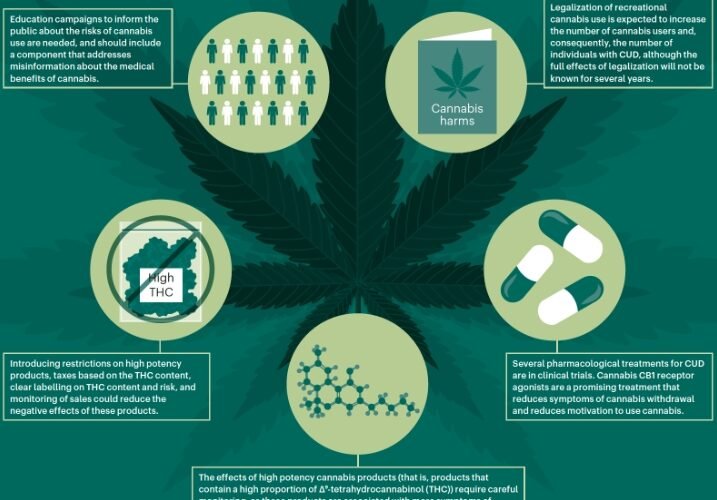
Indivior PLC has encountered a significant setback in its efforts to develop a treatment for cannabis use disorder (CUD). The company’s Phase 2B clinical trial, conducted in collaboration with Aelis Farma, failed to meet its primary and secondary endpoints. This trial aimed to evaluate the efficacy and safety of AEF0117, a potential treatment for individuals with moderate to severe CUD. Despite the disappointing results, Indivior remains committed to understanding and addressing the complexities of cannabis addiction.
Trial Results and Implications
The Phase 2B trial of AEF0117 involved participants with moderate to severe cannabis use disorder. The primary goal was to reduce cannabis use to one day or less per week. Unfortunately, the trial did not achieve this target, nor did it meet secondary endpoints such as complete abstinence or reduced use to two days or less per week. These results indicate that AEF0117 may not be effective in its current form for treating severe CUD.
The failure to meet these endpoints has significant implications for Indivior’s strategy. The company had hoped that AEF0117 would provide a new treatment option for those struggling with cannabis addiction. However, the lack of efficacy in this trial suggests that further research and development are needed. Indivior must now decide whether to continue investing in AEF0117 or to explore alternative approaches to treating CUD.
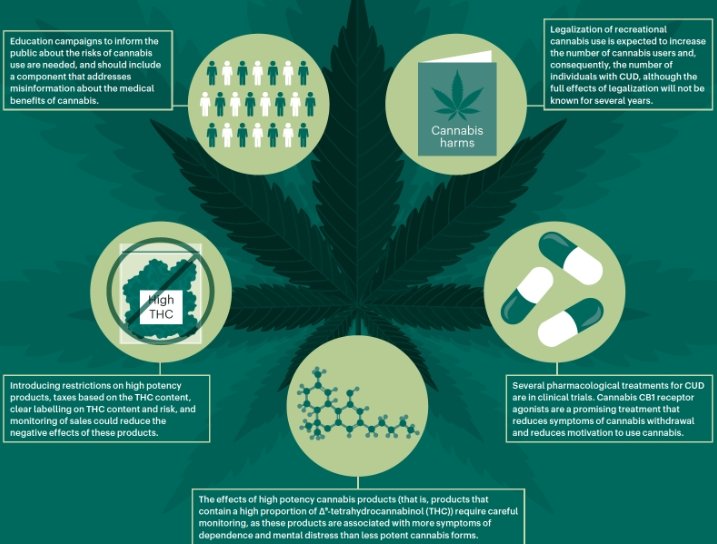
Despite the setback, the trial provided valuable insights into the challenges of treating cannabis use disorder. The data collected will help researchers better understand the condition and identify potential subpopulations that may respond differently to treatment. This knowledge is crucial for developing more targeted and effective therapies in the future.
Financial and Strategic Impact
The trial’s failure has financial and strategic repercussions for Indivior. The company had invested significant resources into the development of AEF0117, and the disappointing results may affect investor confidence. Indivior’s stock price could be impacted as the market reacts to the news. Additionally, the company must reassess its pipeline and allocate resources to other promising projects.
Indivior’s collaboration with Aelis Farma included an option to license the global rights to AEF0117. Given the trial’s outcome, Indivior has decided not to exercise this option at this time. This decision allows the company to conserve resources and focus on other areas of its portfolio. However, it also means that Aelis Farma will need to seek other partners or funding sources to continue developing AEF0117.
The setback underscores the inherent risks in pharmaceutical development. Despite rigorous testing and promising early results, many potential treatments fail to demonstrate efficacy in later-stage trials. Indivior’s experience with AEF0117 highlights the importance of resilience and adaptability in the face of such challenges.
Future Directions and Opportunities
While the trial results are disappointing, they also present an opportunity for Indivior to refine its approach to treating cannabis use disorder. The company can leverage the data from this trial to design more effective studies and identify new therapeutic targets. Indivior’s commitment to addressing addiction remains strong, and the lessons learned from this setback will inform future research efforts.
Indivior may also explore partnerships with other companies or research institutions to advance its understanding of CUD. Collaborative efforts can bring together diverse expertise and resources, increasing the likelihood of success. By working with external partners, Indivior can accelerate the development of new treatments and expand its impact on the field of addiction medicine.
In the meantime, Indivior continues to focus on its broader mission of developing innovative treatments for addiction. The company has a robust pipeline of products targeting various substance use disorders, including opioid addiction. By maintaining a diversified portfolio, Indivior can mitigate the risks associated with any single project and continue making progress in the fight against addiction.